In our modern world, where portable electronics, electric vehicles, and renewable energy sources dominate, the unsung hero powering these advancements is the lithium-ion battery. From smartphones to grid-scale energy storage systems, lithium-ion batteries have become an indispensable part of our daily lives. But what exactly makes them tick? How are they constructed, and why are they so prevalent? Let’s delve into the intricate world of lithium-ion batteries.
The Foundation: Fundamental Components
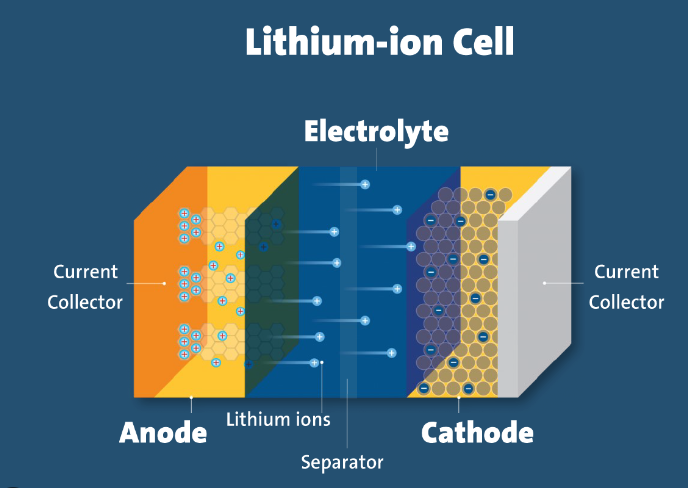
Anode and Cathode
At the heart of every lithium-ion battery lies the anode and cathode, crucial components responsible for the battery’s function. The anode, typically composed of graphite, serves as the site where lithium ions are stored during charging. On the other hand, the cathode, often containing lithium metal oxides like lithium cobalt oxide or lithium iron phosphate, accepts these ions during discharge.
Separator
Separating the anode and cathode is a permeable, micro-porous separator. This critical component prevents electrical short circuits while allowing the flow of lithium ions between the electrodes. Typically made of polymer materials, the separator acts as a safety feature, preventing overheating and potential hazards.
Electrolyte
The electrolyte, a conductive solution containing lithium salts dissolved in a solvent, facilitates the movement of ions between the anode and cathode. This conductive medium is essential for the flow of ions during charging and discharging cycles.
The Chemistry of Charge
The operation of a lithium-ion battery is deeply rooted in chemical reactions. During charging, lithium ions move from the positive electrode (cathode) to the negative electrode (anode) through the electrolyte and separator. This movement is the result of an external electrical current applied to the battery.
Conversely, during discharge—when the battery powers a device—lithium ions migrate back to the positive electrode through the electrolyte, generating an electric current that can be harnessed to power various devices.
Manufacturing Process
Electrode Production
The manufacturing process begins with the production of electrodes. Thin layers of active materials (like lithium cobalt oxide or graphite) are coated onto metal foils. These coated foils serve as the positive and negative electrodes of the battery.
Cell Assembly
The electrodes, along with the separator, are assembled into a cell. This process involves carefully stacking and winding these components to ensure efficient ion flow and electrical conductivity.
Sealing and Formation
Once the cell is assembled, it is sealed to prevent leaks and contamination. Subsequently, a process known as “formation” occurs, where the battery undergoes initial charging and discharging cycles to stabilize its performance.
Testing and Packaging
After formation, batteries undergo rigorous testing to ensure quality and performance standards are met. Once verified, the batteries are packaged, ready to be integrated into various applications.
Advancements and Challenges
Advantages
The widespread adoption of lithium-ion batteries can be attributed to their high energy density, long cycle life, and relatively low self-discharge rate. These qualities make them suitable for diverse applications, from portable electronics to electric vehicles.
Challenges
However, challenges persist. Safety concerns, such as the potential for thermal runaway and fire hazards, remain a focal point for ongoing research and development. Additionally, the reliance on finite resources like cobalt and nickel raises concerns about sustainability and cost.
Future Innovations
As technology progresses, researchers and engineers are exploring alternative materials and battery designs to address current limitations. Advancements in solid-state electrolytes, which offer improved safety and energy density, show promise for the next generation of batteries. Moreover, the development of lithium-sulfur and lithium-air batteries may significantly increase energy density and reduce reliance on rare materials.
Conclusion
Lithium-ion batteries have revolutionized the way we live, powering our devices and propelling the electric vehicle industry forward. Their construction, rooted in complex chemistry and precise engineering, embodies the fusion of scientific innovation and practical application. However, as demands for energy storage escalate, ongoing research and development are imperative to overcome current limitations and pave the way for more efficient, safer, and sustainable energy storage solutions.
As we embark on this journey towards better energy storage, the evolution of lithium-ion batteries stands as a testament to human ingenuity, pushing the boundaries of what is possible in the realm of energy technology.
